Chemical Engineering
Design, construct, and analyze process machinery including reactors, boilers, and pumps. Solve heat transfer problems and design control systems.
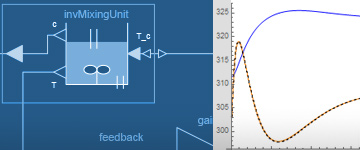
Controlled Mixing Unit
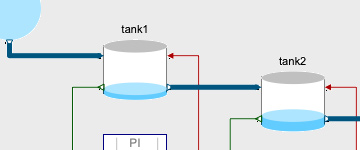
An important control problem is to design controllers for nonlinear systems using model inversion control. The following example uses an InverseBlockConstraints component to easily construct inverse models. It also uses the Modelica_Synchronous Library to automatically discretize the continuous-time nonlinear feedforward controller.
Chemical Reactions
When doing a chemical experiment, it is useful to know how much product you will obtain when you mix certain amounts of reactants. This is particularly useful in real-world settings, such as in chemical production or chemical analysis. In this example, you will learn how to predict the outcome of a reaction, given the initial amounts of reactants and the ratio between the products and substrates.
Chemical Equilibrium
Most chemical reactions are not irreversible and in fact go in both directions. However, all reversible reactions reach a steady state in which the concentrations of the substrates within the reaction become constant. In this example, you will learn to model a reversible chemical reaction and analyze the dynamics of the reaction. You will learn about equilibrium constants and reaction rates.
Heterogenous Equilibrium
Heterogenous equilibrium refers to reversible reactions where the substrates of the reactions are in different phases. One of the most useful types of such reactions is where a solid is dissolved in an aqueous solution. In this example, you will learn about heterogenous equilibrium and understand the concept of solubility.
Rate Laws
Chemical reactions occur at different rates and vary widely in the speeds at which they occur. Some reactions occur very fast; for example, an explosion or combustion of fuel in a race car. In this example, you are going to work with chemical kinetics and the study of rates of chemical processes. You will learn how to calculate rate, reaction order and half-life of a reaction.
Wolfram System Modeler
Try
Buy
System Modeler is available in English
and Japanese
on Windows, macOS & Linux »
Questions? Comments? Contact a Wolfram expert »