Learn How Melting and Boiling Points of Chemicals Vary
The Wolfram Language has the entity type "Chemical", which covers a wide variety of chemical species, from metal carbonyls to polyprotic acids to elemental allotropes. Chemical entities are distinct from Molecule objects because they are backed with curated data from the Wolfram Knowledgebase.
Find experimental properties for a chemical.
Find the standard chemical name of a chemical.
Using "Chemical" entities, you can explore how different properties scale with the number. The melting and boiling points of the alkanes is a typical example. Start by using the entity class ![]() . There are 433 entities in this class.
. There are 433 entities in this class.
Use RandomEntity to get 3D plots for 20 random alkanes.
Make a histogram to visualize the distribution of masses.
The breadth of knowledge available for "Chemical" entities allows you to see relationships between different properties. Here, look at how the melting and boiling points of alkanes vary with the number of carbon atoms.
First, define an EntityFunction that returns the number of carbons and use this function directly in EntityValue alongside built-in properties.
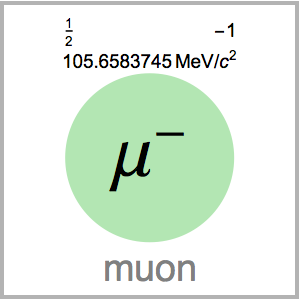
You can see that ![]() and
and ![]() have unusually high melting points for their size. The ball-and-stick structures show they also have a high number of bridged rings.
have unusually high melting points for their size. The ball-and-stick structures show they also have a high number of bridged rings.
You can create a FilteredEntityClass to represent only acyclic alkanes, using an EntityFunction to select entities with the formula  .
.
Now use the filtered class directly in an EntityValue call.
The plot looks much cleaner when run with the filtered data.
The dependence on size is even more pronounced when plotting the boiling points.