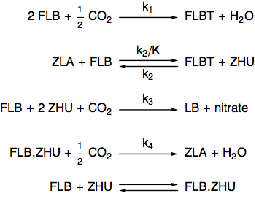
Chemical Reactions
Model a chemical process of two species, FLB and ZHU, that are continuously mixed with carbon dioxide.
The inflow of carbon dioxide per unit volume is denoted as follows.
| In[1]:= | X |
Specify the rate equations for each of the reactions.
| In[2]:= |  X |
The governing equations for the rate of change of concentrations of each chemical species depends on the rate equations and the reactions.
| In[3]:= |  X |
The last reaction represents an equilibrium reaction.
| In[4]:= | X |
Define the reaction parameters for each of the reactions.
| In[5]:= | X |
Specify the initial concentrations of each species.
| In[6]:= | X |
Solve and visualize the change in concentration of the species over time.
| In[7]:= | X |
| In[8]:= | 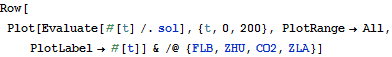 X |
| Out[8]= | 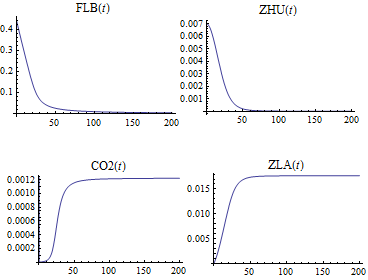 |