Equilibrio químico
Modelado de una reacción reversible
Utilice diversos componentes incorporados de una reacción, tales como sustratos y tipos de reacción, para modelar la reacción reversible. Conecte estos componentes y defina sus valores iniciales.
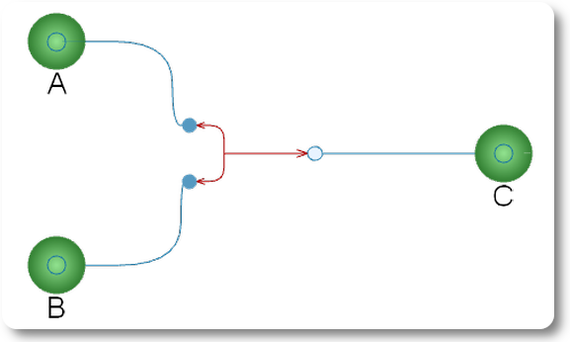
Una reacción de equilibrio con dos reactivos A y B y un producto C.
Analysis
Calcule las velocidades directa e inversa y analice la dinámica de la reacción reversible para diferentes concentraciones iniciales de los sustratos.
Análisis
Use Wolfram Language para realizar análisis personalizados.
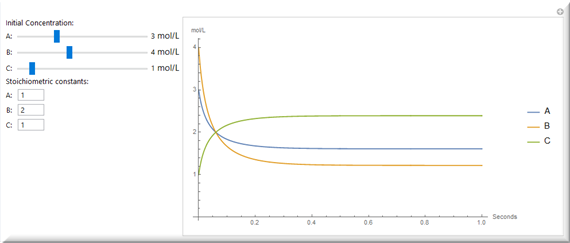
Variación de la composición química a medida que la reacción avanza.
Wolfram System Modeler
Probar
Comprar
System Modeler está disponible en inglés
y japonés
para Windows, macOS y Linux »
¿Preguntas? ¿Comentarios? Contacte a un experto de Wolfram »