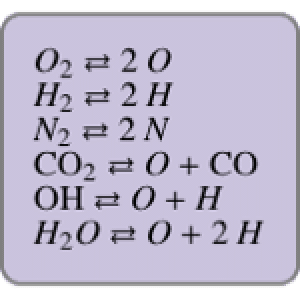Find Chemical Equilibrium for Networks of Reactions
Chemical reaction systems that have equilibrium can typically be directly modeled as a polynomial system of equations. These problems are defined by conservation and reaction equations. The example below is from combustion chemistry and models the burning of fuel in a combustion chamber such as in a car engine. The goal is to find out what chemical components and compounds are in the combustion chamber at various stages. The system of reactions being studied is given by 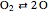 ,
, 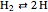 ,
, 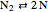 ,
, 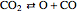 ,
, 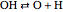 ,
, 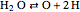 ,
, 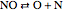 .
.
Introduce a variable for the quantity of each component. Here 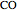 is treated as a component, since
is treated as a component, since 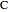 does not occur on its own.
does not occur on its own.
 |
Also introduce a variable for the quantity of each compound.
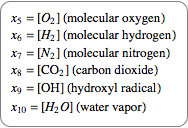 |
| In[1]:= | X |
If you assume a closed chemical system, quantities are conserved, which leads to conservation equations shown in their chemical and algebraic notation.
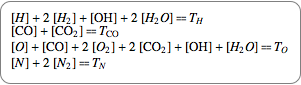 |
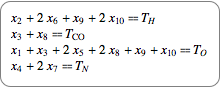 |
| In[2]:= | X |
Give the equations for equilibrium.
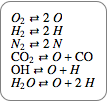 |
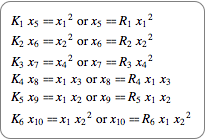 |
| In[3]:= | X |
Give parameter values.
| In[4]:= | 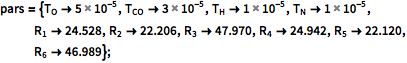 X |
Solve the system over the reals, and select only non-negative solutions.
| In[5]:= | X |
| In[6]:= | X |
| Out[6]= |
Compute a higher-precision solution.
| In[7]:= | X |
| In[8]:= | X |
| Out[8]= |  |